Monomers- Definition, Examples, and Structure
Introduction
A monomer is a molecule that can be polymerized or joined with other molecules to form a macromolecule. The most common type of monomers in use is the sugars glucose and fructose. Monomers will combine to make polymers, which are long chains of repeating units. Polyesters (plastic), nylon, and proteins all have monomers as their building blocks. In this post, we’ll delve deeper into monomers. However, should you choose to skip this guide, our professional writers for hire are ready to cover you by acing that assignment for you.
How are Monomers Classified?
Monomers are classified into two categories: natural and synthetic. These two classifications are based on the origin of the monomers. Natural monomers are derived from natural resources, and synthetic monomers come about by chemical synthesis or a reaction between two chemicals.
Natural Monomers
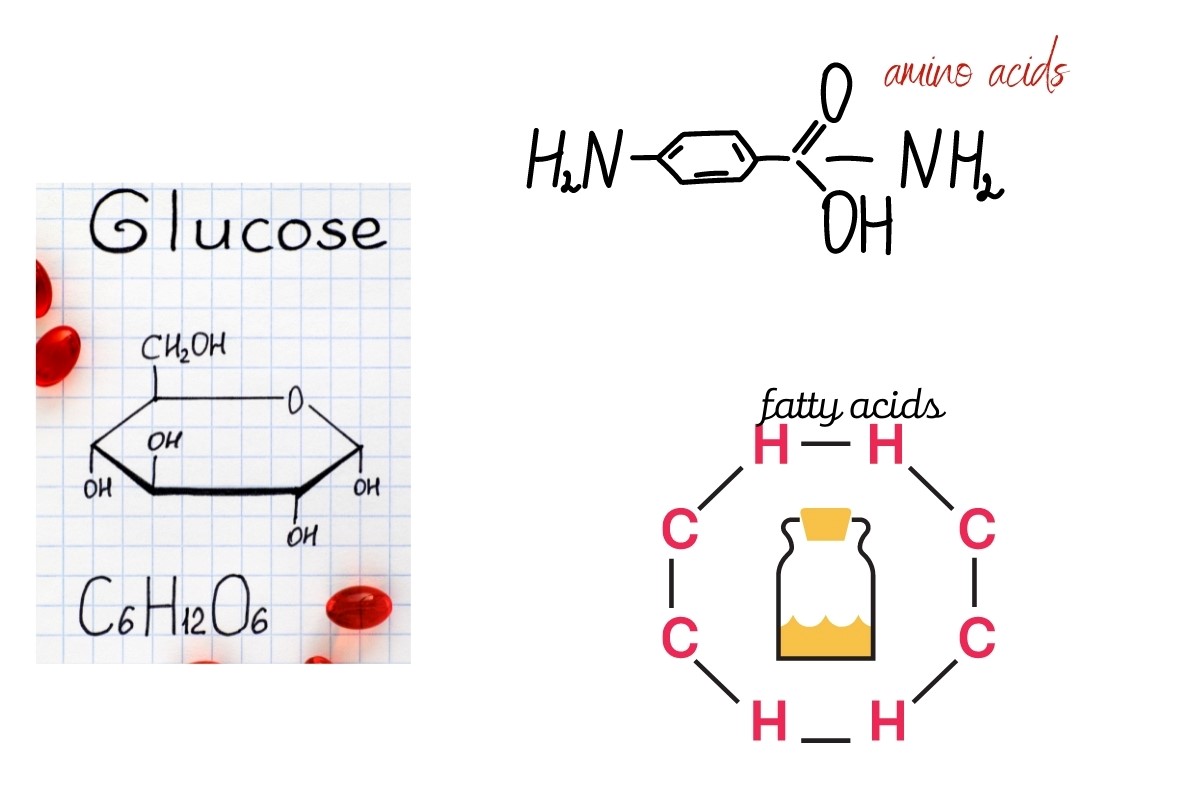
Natural monomers are those that come from natural resources and are biodegradable. They include Monosaccharides, Nucleotides, Isoprene, Amino acids, fatty acids, and alcohol.
Monosaccharides
Monosaccharides are a class of monomer that is composed of only one sugar unit. When they polymerize, the result is called a saccharide. Monosaccharides are classified according to their number of carbon atoms:
There are two types of monosaccharides: aldoses, which have an aldehyde group, and ketones. The most common monosaccharide is glucose, which is found in vegetables and fruits.
Sugars such as fructose are also monosaccharides, but they have a ketone group. Other monosaccharides include galactose and ribose, both of which are found in nucleotides.
Structure
Monosaccharides are classified according to their number of carbons:
- Aldoses, have an aldehyde group. These include glucose and fructose (found in vegetables and fruits).
- Ketones, have a ketone group. These include galactose (found in nucleotides) and ribose (found in nucleotides).
Monosaccharides have a backbone of carbon atoms that can be cyclized or linear. When they are linear, they have a straight backbone. When cyclized, the molecules in the ring behave like double or triple bonds because of resonance.
You can expand your knowledge by checking our exclusive guide on the structures of basic macromolecules.
What are the Properties of Monosaccharides?
The following are the general properties of monosaccharides:
- They have a sweet taste
- Monosaccharides contain a single sugar molecule
- They are soluble in water
- Monosaccharides can be used as a fuel for cellular respiration
- When they polymerize, the result is called a saccharide.
- Monosaccharides are classified according to their number of carbon atoms.
Nucleotides
Nucleotides are made of a pentose-sugar molecule, at least one phosphate group, and a nitrogenous base. They are the building blocks for DNA and RNA molecules.
Nucleotides can be classified into four categories:
- Purines: adenine (adenine) and guanine (guanine).
- Pyrimidines: cytosine, thymine, and uracil.
- Sugar phosphate backbone: ribose or deoxyribose, found in RNA and DNA, respectively.
- Nonstandard nucleotide: inosine.
Purines
Purines are a type of nucleotide that have two rings. Adenine and guanine, which come from the same molecule as adenosine triphosphate (ATP), are examples.
When these two types of nucleotides bond, they form a pyrimidine. Purines are either adenine or guanine. When these two nucleotides bond with a pyrimidine, they form an ATP molecule.
Pyrimidines
Pyrimidines are a type of nucleotide that have three rings. Cytosine, thymine, and uracil come from pyrimidine triphosphate (TP). When these nucleotides bond, they form a purine.
These monosaccharides are either cytosine, thymine, or uracil. When these three nucleotides bond with a purine, they form the pyrimidine triphosphate molecule.
The sugar-phosphate backbone can be ribose or deoxyribose, found in RNA and DNA, respectively. When these nucleotide molecules bond, they form a DNA molecule.
Inosine is the only nonstandard nucleotide, and it has no backbone or sugar-phosphate groups. Its only role is to allow the DNA molecule to fold.
- It can be found as a substituent in RNA, where it forms Gs and Gc. Or a substituent in DNA, where it forms Cs and Us.
How is the Chemical Bonding of Nucleotides?
The chemical bonding of nucleotides is held together by hydrogen bonds. It can be classified into two groups: intramolecular and intermolecular.
Intramolecular bonding is when the molecules in a single molecule are held together by covalent bonds, noncovalent bonds, hydrogen bonding, or metal-metal interactions.
Our exclusive guide on the fluid mosaic model can help you visualize the cell and cell components better!
Amino Acids
Amino acids are organic compounds that contain both amine (-NH) and carboxylic acid (COOH). Their general formula is R-CH(NH2)-COOH, and they are the building blocks of proteins.
The R is the group that determines the type of amino acid: either a side chain or the amino group. The side chain can be either an amine, a ketone, or an acid.
The three main types of amino acids are:
- Neutral amino acids have a side chain that is not an amine or acid.
- Basic amino acids have a side chain that is an amine or acid.
- Acidic amino acids have a side chain that is an amine or acid.
Amino acids are classed according to their side chain, either an amine, a ketone, or an acid.
- Neutral amino acids have no side chain and are glycine, alanine, valine, leucine, isoleucine, and phenylalanine.
- Basic amino acids have an amine or acid side chain that has been modified by adding a hydroxyl (-OH) group. The side chain can be either histidine, lysine, arginine, or aspartic acid.
- Acidic amino acids have an amine or acid side chain that has been modified by adding a carboxyl (-COOH) group. The side chain consists of asparagine, glutamic acid, and glutamate.
You may want to familiarize yourself with the most used biochemical terms!
Fatty Acids and alcohol
Fatty acids are organic molecules that contain a long hydrocarbon chain. They can be saturated or unsaturated, and the carbon chains typically have an even number of carbon atoms.
Alcohols are organic compounds containing an -OH group on a hydrocarbon chain, typically one of the following: alkanes, alkenes, or alkynes.
Alcohol and fatty acids are the monomers that form lipids. They are made of long-chain hydrocarbons.
Lipids are also known as fats and oils. They can be classified into saturated, unsaturated, and polyunsaturated.
When alcohol reacts with the fatty acids, they form triglycerides. When the fatty acid reacts with glycerol, they form a molecule known as a lipoprotein or fat cell membrane.
Isoprene
Isoprene is a molecule that contains the elements hydrogen, carbon, and oxygen. It can be found as monomers or polymers of up to about 800 units long.
This monomer is the main precursor of natural rubber and chlorophyll, made up of about 200-800 isoprene units.
Liquid isoprene can be obtained through fractional distillation of natural gas, which also produces hydrocarbon butane.
It can also be found in pine trees, and isoprene emissions are greater during daytime hours than at night.
Properties of Isoprene
Isoprene is a monomer that can be found in natural gas as well as pine trees. Some of its properties are:
- It is liquid
- Isoprene has a strong smell.
- It is a polymer that can be up to about 800 units long.
- Isoprene is a hydrocarbon with the formula C(H) H. This means that it is a hydrocarbon in which single bonds join all the carbon atoms.
- Its molecules can react with other substances to form isoprenoids.
- This monomer can be found in natural gas, and pine trees and has the odour of strong gasoline.
Isoprene is used for making rubber, which is an important industrial material. It can also be used as a natural insecticide to produce chlorophyll, which is an important part of photosynthesis.
Our exclusive guide on functions of the basic macromolecules can be a useful resource
Synthetic Monomers
Synthetic monomers are compounds that have been created in a laboratory. They can be made from either natural or non-natural sources, and they can be composed of monomers, polymers, or both.
Some synthetic monomers are:
- Acrylic acid
- Methacrylic acid
- Vinyl acetate monomer
- Styrene monomers.
The most common synthetics are made from petrochemicals. Polymers are synthetic monomers that have reacted together to form chains of repeating units called polymeric chains.
Characteristics of synthetic monomers
Some properties of synthetic monomers include:
- Chains can be long and composed of repeated units.
- They can be either natural or non-natural and are composed of different types of monomers, polymers, or a combination of both.
- They may have different physical and chemical properties than natural monomers.
- Depending on the synthetic, they may be soluble or insoluble in water.
- They can sometimes dissolve in organic solvents.
You may be interested in Anabolism
Summary
Monomers are the building blocks of polymers. They can be small molecules or large and complex structures like proteins, DNA, and carbohydrates. The monomer is the smallest unit that contains a repeatable pattern in polymer formation (i.e., it’s what you might call “the same thing over and over again).
A key point to remember when thinking about these smaller units is that they all contain carbon atoms – which means your body uses them every day! To learn more about monomers, read more of our blogs on macromolecules. This way, you will understand how monomers work with other types of molecules in forming larger molecules. However, in case you still do not know where to start, our professional writers will be happy to help with that assignment. Just place an order with us today.